Why do things freeze and melt?

by Matteo
At a call on the 31 March we have been asked to provide some material to help learning about the states of matter! This is a great topic, some of us (Valentina) have spent at least a decade studying state transitions involving solids!.
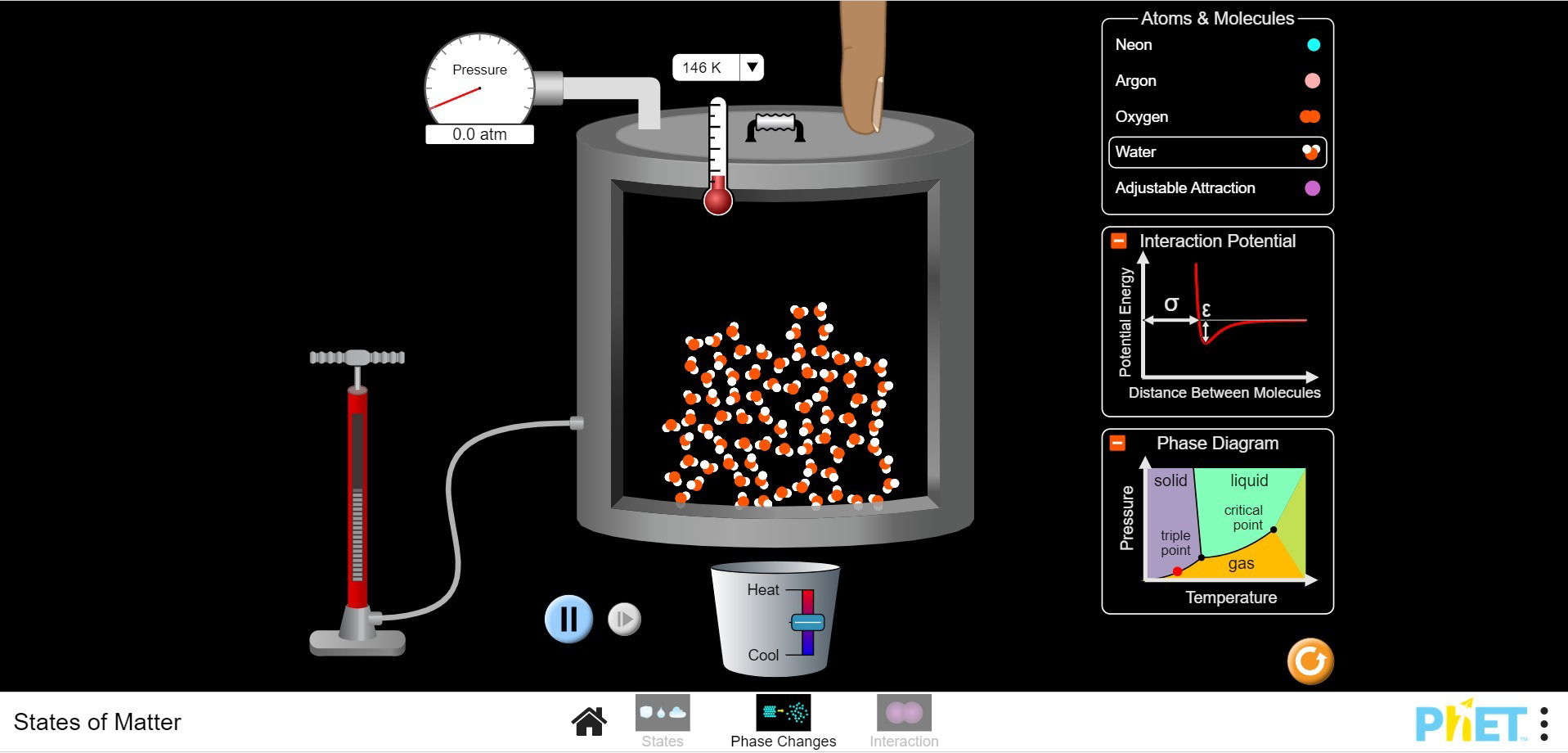
- Depending on temperature and pressure, any material can become solid, liquid or gas. This is what we call the phases (or states) of matter. The University of Boulder Colorado produced great interactive simulations (PhET simulations) to understand maths and physics. Go play with the simulations on phases of matter (opens in a new page) to see how pressure, temperature and type of molecule together determine whether matter will behave like a solid, a liquid or a gas!
- [For the younger ones] Here is a nice video explaining the difference between solid, liquid with popsicles, juice and vapour.
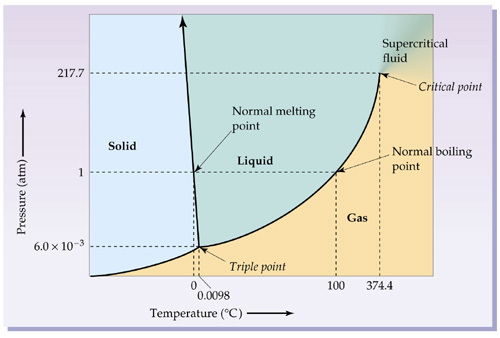
- [For the older ones] So, the colder it is, the more likely is is things will transition from gas to liquid, and from liquid to solid. However, pressure has an effect too! For instance, it is possible to squeeze gas molecules together until they are forced to behave like liquid. So the phase of matter depends on a combination of temperature and pressure, which we represent in what is called a phase diagram.
- How does water freeze? When water molecules are liquid they wobble and bump into each other randomly, but the more you cool them, the more it is likely that some of them will prefer to arrange themselves in a tiny crystal. When a tiny crystal forms, other surrounding water molecules will want to attach to it, which makes the crystal grow. We call this phenomenon nucleation. In the videos below you can see some cool examples exploiting nucleation to make ice!
- A beautiful example of nucleation are snowflakes. A snowflake starts forming when few molecules of water in a cloud get together. When it is very cold, they organise themselves into a tiny crystal, so light that it can float in the air. One by one, other water molecules organise themselves around this tiny crystal, making it grow. Depending on the shape of the initial crystal, and on atmospheric conditions, snowflakes can have lots of different shapes, see the examples in the video below!
- So, we understand how water freezes very well then, right? Enter the Mpemba effect: surprisingly, hot water apparently freezes faster than the cold one. Watch what happens when it's really cold outside, and you throw hot water in the air (it never gets cold enough for this in the UK, but in any case do not try this without an adult!)
The reasons for the Mpemba effect have been unclear for a long time, to the point that in 2012 the Royal Society of Chemistry organised a competition to explain this phenomenon. 22'000 people submitted their explanation!
- Here are some links to some more cool experiments with phase transitions discussed during the chat:
